ICSI or Embryo Culture
$0.00
SKU: MC2015
PACKAGING: 1
HUCHUANG LOW WALL PETRI DISH
Designed for ICSI or embryo culture.From the selection of raw materials to the production of finished products, the series of Petri dish products of HUCHUANG IVF laboratory have passed strict quality certification and safety testing, providing a safer culture condition and development environment for gametes and embryos.
⌀ 60mm,200 Pcs/Case
PRODUCTS FEATURES
Made of Medical Grade USP VI Raw Materials:Polystyrene (PS)
Surface treated options for a consistent hydrophilic surface
Full batch control of all components in the final product for full traceability
Certificates of conformity available for every lot
SAFETY AND QUALITY ASSURANCE
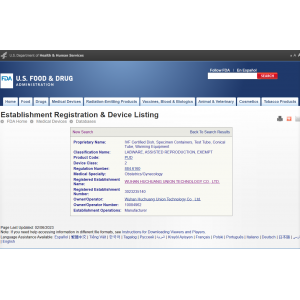
FDA Class II medical device registration
Sterile (irradiation, SAL 10-6)
Non-pyrogenic, Endotoxin (LAL < 0.5 EU/Device)
Acute systemic toxicity, genetic toxicity, non-toxic
Routine three items through biological evaluation(Cytotoxicity, Sensitization, Intradermal irritation)
Dnase/Rnase free
Human sperm survival assay, no sperm toxicity
Mouse embryo assay, MEA≥80%
Weight: 0.2 (kg)